FDA allows the commercialization of UM’s sound-wave therapy for liver cancer
- Healthcare
- October 23, 2023
A medical services startup, helped to establish by College of Michigan workforce, got government endorsement to showcase its harmless procedure to treat destructive growths in the liver, said the organization and UM.
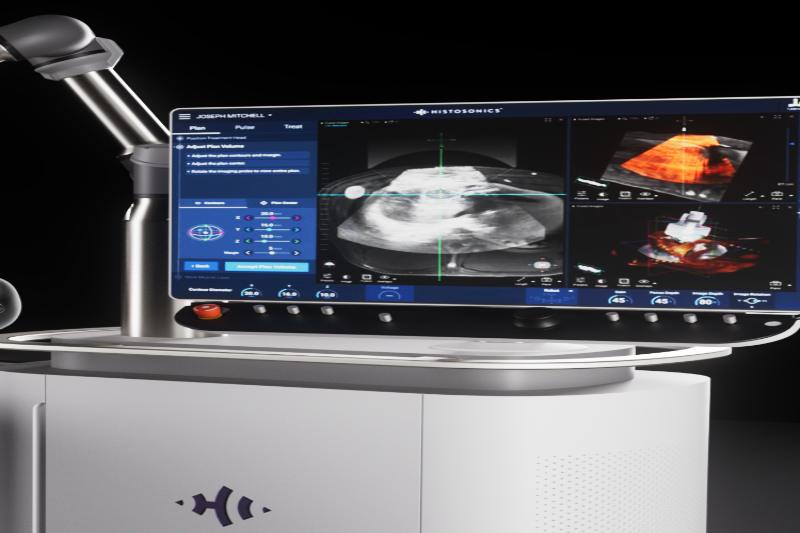
The U.S. Food and Medication Organization supported the market approval this long stretch of the clinical stage that depends on sound waves to separate growths in people for liver medicines. The innovation was created by the firm HistoSonics.
The organization is settled in Minneapolis, while its high level innovative work is situated in Ann Arbor.
‘This is HistoSonics’ most meaningful milestone to date and represents over two decades of tireless efforts, from its inception at the University of Michigan in 2001,” said Mike Blue, president and Chief of HistoSonics, in a proclamation. The firm has been adding staff to showcase clinical offices and train doctors Blue said.
HistoSonics was helped to establish by UM designers and specialists, as indicated by the firm.
The FDA endorsement implies HistoSonics can market and sell its histotripsy conveyance stage, called Edison, to clinics and clinical experts for use in liver medicines.
Histotripsy works by utilizing designated ultrasound waves to frame microbubbles inside the cancer. As the air pockets structure and breakdown, they make the mass fall to pieces, killing cancer cells and passing on the trash to be tidied up by the resistant framework.
“Histotripsy is an exciting new technology that, although it is in early stages of clinical use, may provide a noninvasive treatment option for patients with liver cancer,” said Mishal Mendiratta-Lala, professor of radiology with Michigan Medicine and principal investigator on the trial at UM. “Hopefully it can be combined with systemic therapies for a synergistic therapeutic effect,” Mendiratta-Lala said in a composed proclamation.
Human preliminaries started in 2021 at the UM Rogel Disease Center and different areas, treated patients with essential and metastatic liver cancers.
FDA approval was based, to some extent, on information in 13 preliminary locales across the US and Europe. Information from the U.S. also, European preliminaries were utilized to survey the clinical security and viability of histotripsy in annihilating designated essential and auxiliary liver growths. The organization will zero in on advertising its Edison Framework in the U.S. also, select worldwide business sectors for liver treatment while growing histotripsy applications into different organs like kidney, pancreas, and others.
‘As a specialist, it’s remunerating to have the option to offer a strategy where we can unequivocally obliterate liver growths without utilizing a surgical blade or needles, ideally empowering the patient’s speedy recuperation while keeping away from specific complexities like careful site diseases or radiation sickness normal with different modalities,’ said Joe Amaral MD, VP Clinical Undertakings for HistoSonics.